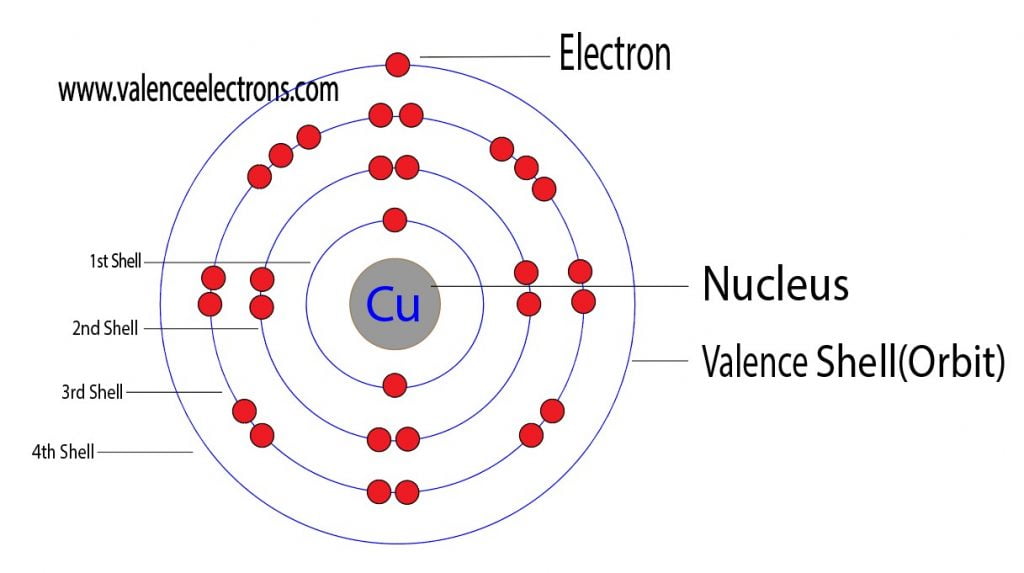
Does Copper Lose Electrons Easily . It’s a chemical dance of. The magnesium can push away its electrons more strongly than copper. It's not that copper is more easily pulled away from. Some metals require more energy than others, e.g. Metals want to lose electrons because the process requires very less energy. Copper(ii) ions are being are being reduced because they are gaining. Copper is somewhat noble, or at least. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. At its core, a redox reaction involves the transfer of electrons between two species. Electrons flow along the wires and through the voltmeter from the. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Magnesium atoms are being oxidised because they are losing electrons; What is a redox reaction?
from valenceelectrons.com
What is a redox reaction? It’s a chemical dance of. Electrons flow along the wires and through the voltmeter from the. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Copper(ii) ions are being are being reduced because they are gaining. Metals want to lose electrons because the process requires very less energy. Magnesium atoms are being oxidised because they are losing electrons; At its core, a redox reaction involves the transfer of electrons between two species. Some metals require more energy than others, e.g. The magnesium can push away its electrons more strongly than copper.
How Many Valence Electrons Does Copper (Cu) Have?
Does Copper Lose Electrons Easily Metals want to lose electrons because the process requires very less energy. What is a redox reaction? Copper(ii) ions are being are being reduced because they are gaining. It's not that copper is more easily pulled away from. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. It’s a chemical dance of. Electrons flow along the wires and through the voltmeter from the. Magnesium atoms are being oxidised because they are losing electrons; Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Metals want to lose electrons because the process requires very less energy. Copper is somewhat noble, or at least. Some metals require more energy than others, e.g. The magnesium can push away its electrons more strongly than copper. At its core, a redox reaction involves the transfer of electrons between two species.
From www.periodictableprintable.com
Periodic Table Elements Lose Or Gain Electrons 2024 Periodic Table Does Copper Lose Electrons Easily It’s a chemical dance of. It's not that copper is more easily pulled away from. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. At its core, a redox reaction involves the transfer of electrons between two species. Magnesium atoms are being oxidised because they. Does Copper Lose Electrons Easily.
From www.slideserve.com
PPT Electrical Theory PowerPoint Presentation, free download ID2425935 Does Copper Lose Electrons Easily Copper is somewhat noble, or at least. Some metals require more energy than others, e.g. Electrons flow along the wires and through the voltmeter from the. Metals want to lose electrons because the process requires very less energy. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Magnesium atoms are being oxidised because they. Does Copper Lose Electrons Easily.
From slideplayer.com
Electrochemistry Movement of Electrons Chemistry of Metal Reactions Does Copper Lose Electrons Easily It’s a chemical dance of. It's not that copper is more easily pulled away from. Electrons flow along the wires and through the voltmeter from the. Copper is somewhat noble, or at least. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. What is a. Does Copper Lose Electrons Easily.
From socratic.org
How can you tell if an element wants to gain or lose electrons? Socratic Does Copper Lose Electrons Easily It's not that copper is more easily pulled away from. It’s a chemical dance of. Magnesium atoms are being oxidised because they are losing electrons; Electrons flow along the wires and through the voltmeter from the. Copper is somewhat noble, or at least. Some atoms have only a few electrons in their outer shell, while some atoms lack only one. Does Copper Lose Electrons Easily.
From www.mooramo.com
Ions of Transition Elements Mooramo Does Copper Lose Electrons Easily At its core, a redox reaction involves the transfer of electrons between two species. The magnesium can push away its electrons more strongly than copper. It’s a chemical dance of. It's not that copper is more easily pulled away from. Copper(ii) ions are being are being reduced because they are gaining. Copper is somewhat noble, or at least. Some atoms. Does Copper Lose Electrons Easily.
From valenceelectrons.com
How to Find the Valence Electrons for Phosphorus (P)? Does Copper Lose Electrons Easily At its core, a redox reaction involves the transfer of electrons between two species. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Some metals require more energy than others, e.g. What is a redox reaction? Some atoms have only a few electrons in their outer shell, while some atoms lack only one or. Does Copper Lose Electrons Easily.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Does Copper Lose Electrons Easily The magnesium can push away its electrons more strongly than copper. Metals want to lose electrons because the process requires very less energy. Copper is somewhat noble, or at least. Electrons flow along the wires and through the voltmeter from the. It's not that copper is more easily pulled away from. Copper still has a higher electronegativity than silver, but. Does Copper Lose Electrons Easily.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Does Copper Lose Electrons Easily At its core, a redox reaction involves the transfer of electrons between two species. The magnesium can push away its electrons more strongly than copper. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Copper(ii) ions are being are being reduced because they are gaining.. Does Copper Lose Electrons Easily.
From 2012books.lardbucket.org
Electrochemistry Does Copper Lose Electrons Easily It's not that copper is more easily pulled away from. Copper(ii) ions are being are being reduced because they are gaining. At its core, a redox reaction involves the transfer of electrons between two species. Electrons flow along the wires and through the voltmeter from the. Magnesium atoms are being oxidised because they are losing electrons; Metals want to lose. Does Copper Lose Electrons Easily.
From www.doubtnut.com
copper ions lose electrons to neutral atoms Does Copper Lose Electrons Easily Copper(ii) ions are being are being reduced because they are gaining. It's not that copper is more easily pulled away from. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Some metals require more energy than others, e.g. What is a redox reaction? The magnesium can push away its electrons more strongly than copper.. Does Copper Lose Electrons Easily.
From ascmag.com
Shot Craft Deep Focus — The Basics of Electromotive Force The Does Copper Lose Electrons Easily The magnesium can push away its electrons more strongly than copper. What is a redox reaction? Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Copper(ii) ions are being are being reduced because they are gaining. Magnesium atoms are being oxidised because they are losing. Does Copper Lose Electrons Easily.
From www.numerade.com
SOLVED List of Materials and their Charge Interaction Based on Does Copper Lose Electrons Easily Copper(ii) ions are being are being reduced because they are gaining. It's not that copper is more easily pulled away from. The magnesium can push away its electrons more strongly than copper. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. Copper is somewhat noble, or at least. Some metals require more energy than. Does Copper Lose Electrons Easily.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Does Copper Lose Electrons Easily Metals want to lose electrons because the process requires very less energy. It's not that copper is more easily pulled away from. Electrons flow along the wires and through the voltmeter from the. The magnesium can push away its electrons more strongly than copper. What is a redox reaction? Copper is somewhat noble, or at least. Some metals require more. Does Copper Lose Electrons Easily.
From baileythorpe.z19.web.core.windows.net
Gain Or Lose Electrons Chart Does Copper Lose Electrons Easily Electrons flow along the wires and through the voltmeter from the. The magnesium can push away its electrons more strongly than copper. Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. At its core, a redox reaction involves the transfer of electrons between two species. It’s a chemical dance of. Copper(ii) ions are being. Does Copper Lose Electrons Easily.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Does Copper Lose Electrons Easily Electrons flow along the wires and through the voltmeter from the. Copper is somewhat noble, or at least. It's not that copper is more easily pulled away from. Magnesium atoms are being oxidised because they are losing electrons; The magnesium can push away its electrons more strongly than copper. What is a redox reaction? Some atoms have only a few. Does Copper Lose Electrons Easily.
From ar.inspiredpencil.com
Copper Orbital Diagram Does Copper Lose Electrons Easily Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. It’s a chemical dance of. Some metals require more energy than others, e.g. Magnesium atoms are being oxidised because they are losing electrons; Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have. Does Copper Lose Electrons Easily.
From www.stonecoldhands.com
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge Does Copper Lose Electrons Easily At its core, a redox reaction involves the transfer of electrons between two species. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. The magnesium can push away its electrons more strongly than copper. It’s a chemical dance of. It's not that copper is more. Does Copper Lose Electrons Easily.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971 Does Copper Lose Electrons Easily Copper still has a higher electronegativity than silver, but copper metal is more easily oxidized. At its core, a redox reaction involves the transfer of electrons between two species. It’s a chemical dance of. Copper(ii) ions are being are being reduced because they are gaining. Magnesium atoms are being oxidised because they are losing electrons; Electrons flow along the wires. Does Copper Lose Electrons Easily.